By now you may have heard that we just released our newest product, the SC-50 MLF Analyzer. We have been working on this for nearly two years, mainly to be sure that the product is easy to use, reliable and accurate. The approach we took was to have the device measure the increase in CO2 pressure that occurs when malic acid is converted to lactic acid, a process we call ‘Biopressure’. This increase in pressure is then converted to an electrical current that the SC-100 or -300 SO2 analyzers can pick up and display. Pretty simple really, but the devil is in the details as they say. And quite honestly we expect the methods (but not the hardware) to improve over time as we learn new ways to make it even faster to use.
 (Chemical Structure of l-Malic Acid)
(Chemical Structure of l-Malic Acid)
They idea of using pressure to measure malic acid in wine is not new in itself. Over 50 years ago, George Kolar of the Australian Wine Research Institute published an article entitled “Manometric Determination of l(—) Malic Acid in Grape Musts and Wines”* that described a method adapted from earlier biochemical research. This method was adopted widely during the 60s, but was eventually displaced by other analytical techniques, most notably the paper and liquid chromatographic methods, and enzymatic spectrometric assays. All of these later methods were either simpler (paper chromatography) or more accurate and suitable for commercial laboratory use. In contrast, the manometric (i.e., based on measuring gas pressure) method, while sensitive and accurate, involved complex glassware and a good deal of professional training to execute.
We believe that the SC-50 incorporates 21st century technology that makes it a pretty good manometric device that is easy to use; the Biopressure agents, reagents, and methods we have developed over the the last 2 years give Dr. Kolar’s technique an entirely new lease on life. You should be able to complete a few or a few dozen tests in 30 minutes, something that would have taken him (or probably his graduate student, poor soul) hours and hours, 50 years ago.
*Am. J. Enol. Vitic 1962 vol. 13 no. 3 99-104 http://www.ajevonline.org/content/13/3/99.abstract


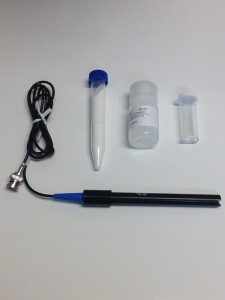
 Do you want to take your winemaking to the next level? We hope you already measure important parameters like free SO2 (sulfites), pH, titratable acidity and malic acid, but residual sugar has always been tricky, expensive, and/or subjective when trying to get a quantitative answer. Now Vinmetrica introduces its NEW Residual Sugar reagent kit. Using the pH meter you already have, you can now get accurate and reliable residual sugar data.
Do you want to take your winemaking to the next level? We hope you already measure important parameters like free SO2 (sulfites), pH, titratable acidity and malic acid, but residual sugar has always been tricky, expensive, and/or subjective when trying to get a quantitative answer. Now Vinmetrica introduces its NEW Residual Sugar reagent kit. Using the pH meter you already have, you can now get accurate and reliable residual sugar data.  How does the Vinmetrica SC-300 SO2 and pH/TA Analyzer Kit compare to other Free SO2 testers on the market? Daniel Pambianchi has done some benchmark comparisons between several Free SO2 testers available. He has created a full report outlining the methods, results and his conclusions. Thank you Daniel for your report. We are happy to share this with our customers.
How does the Vinmetrica SC-300 SO2 and pH/TA Analyzer Kit compare to other Free SO2 testers on the market? Daniel Pambianchi has done some benchmark comparisons between several Free SO2 testers available. He has created a full report outlining the methods, results and his conclusions. Thank you Daniel for your report. We are happy to share this with our customers. 